PRODUCT IDENTIFICATION
28002-18-8 (Disodium)
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions.
APPLICATIONS
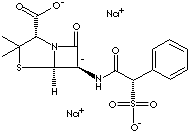
Sulbencillin is a semisynthetic penicillin which has the active core body of 6-aminopenicillanic acid with modification of sulfonic side chain at alpha position. Suncillin (N-sulfonicpenicillin) is an analogue of sulbencillin. The chemical designation is (2S,5R,6R)-3,3-dimethyl-7-oxo-6-((2R)-2-phenyl-2- sulfonatoacetylamino)- 4-thia-1-azabicyclo (3.2.0)heptane-2-carboxylic acid.
Some modified penicillins at alpha position include:
- alpha-Sulfonatopenicillins
- Sulbenicillin Base (CAS #: 41744-40-5)
- Sulbenicillin Disodium (CAS #: 28002-18-8)
- Suncillin
- Carbenicillin Base (CAS #: 4697-36-3)
- Indanyl-carbenicillin (CAS #: 35531-88-5)
- Carbenicillin Indanyl Sodium
- Carbenicillin Potassium
- Carbenicillin Disodium (CAS #: 4800-94-6)
- Carbenicillin Phenyl Sodium or Carfecillin Sodium (CAS #: 21649-57-0)
- Carfecillin Base (CAS #: 27025-49-6)
- Ticarcillin Base (CAS #: 4697-14-7 or 34787-01-4)
- Ticarcillin Cresyl Sodium
- Ticarcillin Monosodiu
- Ticarcillin Disodium (CAS #: 74682-62-5)
- Apalcillin (CAS #: 63469-19-2)
- Azlocillin (CAS #: 37091-66-0 or 37091-65-9)
- Azlocillin Sodium
- Furbenicillin
- Furbenicillin Sodium
- Mezlocillin Base (CAS #: 51481-65-3)
- Mezlocillin Sodium
- Piperacillin Base (CAS #: 61477-96-1)
- Piperacillin Sodium (CAS #: 59703-84-3)
- Pirbenicillin (CAS #: 55975-92-3)
APPEARANCE
IDENTIFICATION
Complies
ASSAY
HEAVY METALS
pH
5.5 - 7.0
MOISTURE
4.0% max